
Amedio CMC Consulting LLC

Drug Substance
• Environmentally friendly synthetic route design
• Process development and optimization
• Process validation
• Control of physical properties (particle size, crystal forms and
polymorphs)
• Physiochemical characterization
• Identification and development of salt forms
• Impurity and genotoxic impurity classification
Drug Product
• Formulation development
– terminal sterilization of injectable solutions
– aseptic processing of lyophilized products
– solid dosage forms (capsules, tablets, ODT, ophthalmic form)
• Bioavailability enhancement of poorly soluble compounds (spray
dried dispersion and hot melt extrusion)
• Support formulation changes during all stages of development
• Packaging design and implementation
Quality Assurance
• Batch record and documentation review and audit
• Maintain internal controls over quality policies, procedures, and
specifications
• Author and review SOPs
• Provide ongoing support and review of Quality Systems
CMC Regulatory Affairs
• Author and review CMC regulatory submissions for pre-IND
meetings, INDs, Annual Reports, Amendments, Type C briefing
books , IMPDs and NDAs
• Participate in face-to-face FDA meetings (pre-IND,
end-of-phase 1, end-of-phase 2 and pre-NDA)
• Review CMO DMFs.
• Provide scientific & regulatory strategy for CMC planning from
proof of concept through commercialization
• Clarification and application of FDA and ICH guidances relevant
to CMC development activities
Manufacturing and Analytical R&D
• cGMP manufacturing scale-up and troubleshooting
• Implement Quality by Design (QbD)
• International and domestic CMO sourcing and management
• Cost management for manufacturing process
• Material sourcing
• Manage technology transfer to and from CMO’s
• Clinical and commercial supply planning and delivery
• Analytical methods development, qualification and validation
• Develop release test methods and specifications
• Design and monitor stability programs
Amedio Consulting provides hands-on expertise that cover CMC services throughout the entire drug development process.
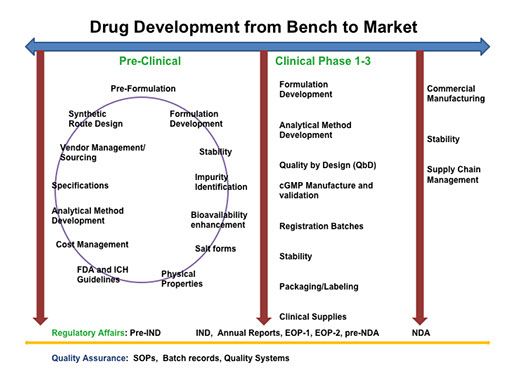
Providing Excellence in Chemistry, Manufacturing and Controls (CMC) Consultation
© 2013 Amedio CMC Consulting LLC | Website: gatestudio.com